Bloggere: Egil Lien, Professor II at Centre of Molecular Inflammation Research (CEMIR) and Mathias Pontus Ørning, PhD candidate.
A number of human-pathogenic bacteria use specialized secretion systems to deliver bacterial effector proteins into host cells. These effector proteins can have many different functions, but the main bacterial goals are believed to be to create a favorable environment for bacterial growth. Thus, inhibition of the host immune responses can dampen bacterial killing and limitations of the infection, tasks that can be enacted by the host innate immune system. Simply said, the bacteria use the effectors to mess up host responses so they can multiply and survive themselves.
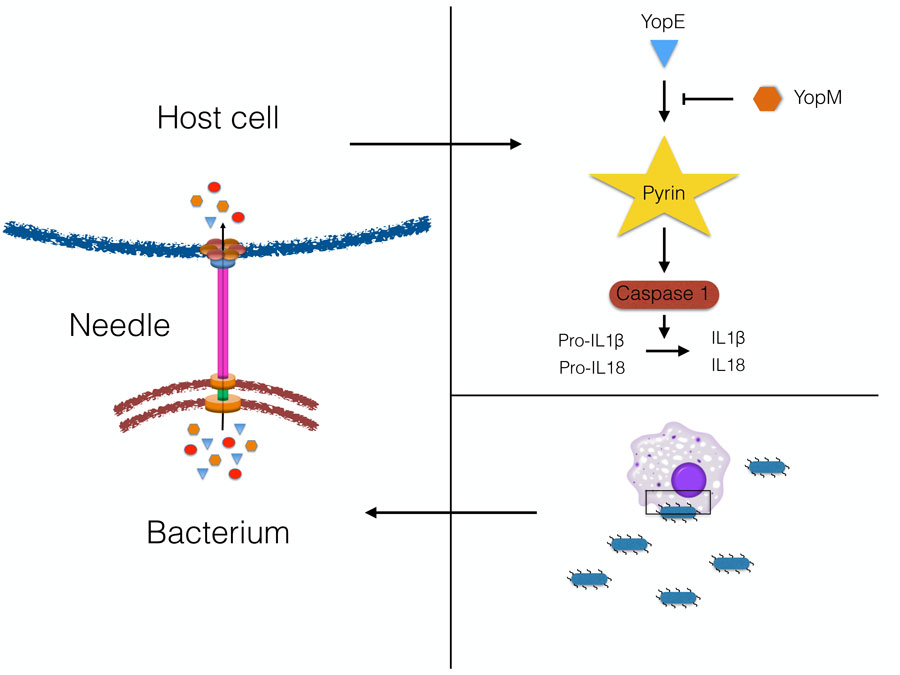
One of the most studied systems is called “type 3 secretion system”, and can be found in various pathogens such as Salmonella, E. coli, Shigella, Yersinia and others. These secretion systems are quite sophisticated nano-machines. A “needle” is protruding from the bacteria and creates a pore in the host cell. Subsequently, the bacterial effectors are translocated into the host cell via these needles and pores (1). It is likely that there is an evolutionary “arms race” between the bacteria and humans, where the bacteria find ways to block immune responses, but the body evolves new ways to circumvent the blockade and detect the infection. We can only hope that the host cells ultimately win!
NTNU-CEMIR researchers have used the “Black Death” plague bacteria – Yersinia pestis – as a model system, and have now discovered how some of these bacterial effectors can block new pathways of the innate immune system. The research has focused on how bacteria manipulate release of host inflammatory signals (cytokines) IL-1b and IL-18, which can induce anti-bacterial responses (2). Yersinia is very effective in dampening release of these cytokines, and one effector, called YopM, blocks activation of a cellular complex called the Pyrin inflammasome, which normally enables release of IL-1b and IL-18. A bacterial strain lacking YopM, attenuated in normal mice, was shown to be virulent in mice lacking Pyrin, thus proving that the mechanism is important for immune function in mammals (3).
Curiously, some people, such as those with the inflammatory disease Familial Mediterranean Fever, have mutant forms of the Pyrin molecule. In these cases, the patients have increased release of IL-1b and IL-18. One hypothesis is that these individuals may have had altered susceptibility to infections (such as the Black Death) throughout history. Can they have been more resistant to infection since they may have increased levels of cytokines, and could this have been a positive selection factor? Perhaps more research can uncover links between Pyrin mutations, historical pandemics and modern infections. In any case, the CEMIR projects have so far uncovered a complex interplay between bacteria and their mammalian hosts, and shown novel aspects of how the body fights to limit infections.
Article about the subject (in norwegian): Slik angriper pest – og slik forsvarer vi oss
1. Ratner, D., Ørning, M.P.A., Lien, E. (2016). Bacterial secretion systems and regulation of inflammasome activation. J Leukocyte Biol. Online Nov 3. pii: jlb.4MR0716-330R.
2. Ratner, D., Ørning, M.P.A., Starheim, K.K., Marty-Roix, R., Proulx, M.K., Goguen, J.D., Lien, E. (2016). Manipulation of IL-1b and IL-18 production by Yersinia pestis effectors YopJ and YopM and redundant impact on virulence. J Biol Chem. 291, 9894-905.
3. Ratner, D., Ørning, M.P.A., Proulx, M.K., Wang, D., Gavrilin, M., Wewers, M., Alnemri, E., Johnson, P., Lee, B., Mecsas, J., Kayagaki, N., Goguen, J.D., and Lien, E. (2016): The Yersinia pestis effector YopM inhibits Pyrin inflammasome activation. PLoS Pathogens 12:e1006035.