Blogger: Catharina de Lange Davies, professor Department of Physics, NTNU
A prerequisite for successful chemotherapy is that the drugs reach its target, and that damage to healthy tissue is limited. However, when drugs are injected into the blood, less than 1% of the drugs accumulate in tumours. Microbubbles combined with focused ultrasound shows great promise in enhancing delivery of nanoparticles and drugs thereby improving cancer therapy. Focused ultrasound and nanoparticles can also be used to temporarily open the blood-brain barrier thereby allowing nanoparticles and drugs to enter into brain tissue, which enables treatment of brain disorders. At the Department of Physics, NTNU, we are using two types of new microbubbles to improve the delivery of nanoparticles and drugs in combination with focused ultrasound.
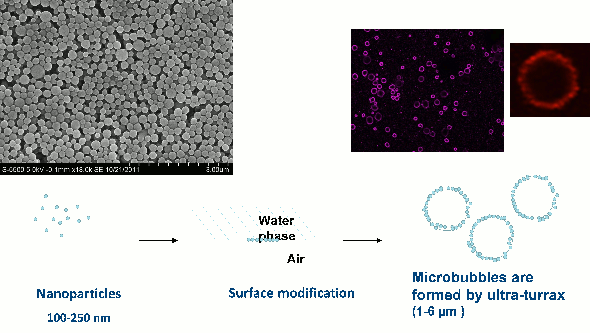
Fig 1: Microbubbles (right) stabilised by nanoparticles (left). The nanoparticles form a shell at the microbubble surface.
SINTEF Materials and Chemistry has developed a new drug delivery system consisting of nanoparticles forming a shell around microbubbles (Fig 1). These nanoparticle-microbubbles have been fluorescently -labelled and injected into the blood in mice with prostate cancer tumours and breast cancer. When combined with optimised ultrasound (1 MHz, MI=0.5, 1000 cycles), the uptake of nanoparticle/dye increased 2.5 times compared to untreated mice.
These encouraging results have been followed up with a small pilot (proof of principle) study where the drug cabazitaxel was incorporated into the nanoparticle. Nanoparticle-microbubble and ultrasound were given twice with one week in between each treatment. The growth of breast cancer tumours was reduced considerably and disappeared, i.e. all tumours went into complete remission. We cured all the mice! We have also shown that these nanoparticle-microbubbles and focused ultrasound temporarily and safely open the blood-brain barrier.
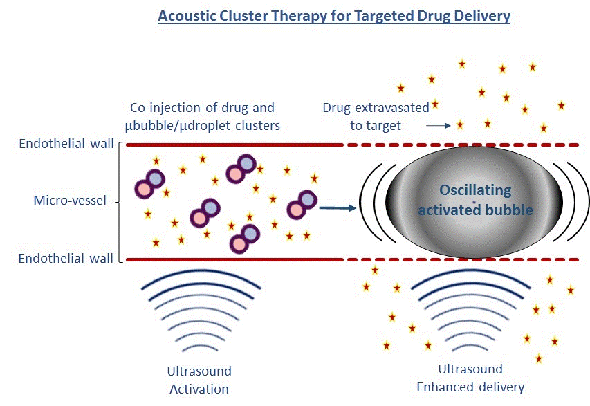
Fig 2: Ultrasound applied to tumors after injecting clusters of microbubbles/microdroplets causes giant oscillating bubbles.
Our other microbubble is developed by one of CIUS’ industry partners, Phoenix Solutions AS. The concept called Acoustic Cluster Therapy (ACT) is based on clusters of microbubbles and microdroplets. After injection, focused ultrasound (using the handheld ultrasound apparatus VScan at 2 MHz, MI=0.4 for 45 sec) is applied to the tumour/disease area whereby the microbubbles transfer energy to the microdroplets, which change from liquid to gas form (Fig 2).
Growing in size to 25 µm, these large bubbles temporarily lodge and block the blood flow at the capillary blood vessel level for up to 10 min. Further exposure to focused ultrasound (500 kHz, 8 cycle every 1 msec for 5 min at MI=0.2) causes these large bubbles to oscillate and increases the ‘leakage’ of drugs or nanoparticles from the blood vessels to the diseased area Fig 2 (4,5). A major advantage for these microbubbles is their large size ensuring close contact between the microbubble and blood vessel wall, and enhancing the mechanical effect on the vessel wall 1000 times compared to regular microbubbles. We have demonstrated that the ACT concept successfully enhances the delivery of macromolecules to prostate tumours growing in mice, by 2-3 times.
Next, we showed that combining ACT with co-injection of the drugs paclitaxel and Abraxane®, induces a very strong increase in the therapeutic efficacy of human prostate tumours in mice. After injecting Abraxane® at a dose used in the clinic combined with ACT, all of the treated mice were alive 120 days after the study started, and 67% were in stable, complete remission. Furthermore, we have shown that ACT can open the blood-brain barrier safely, allowing macromolecules to enter into the brain tissue.
These encouraging preclinical results will now been taken to the clinic. ACT will go into clinical trial in 2018, and at St. Olavs Hospital we will start a clinical study of patients with inoperable pancreatic cancer. The patients will receive standard chemotherapy, diagnostic microbubbles and focused ultrasound using new 3D ultrasound probes with two frequencies. Hopefully, these clinical trials will lead to new clinical practice and improved cancer therapy.
The projects are supported by Helse Midt-Norge and the Research Council of Norway.
Further reading:
- Mørch Y, et al, Nanoparticle-stabilized microbubbles for multimodal imaging and drug delivery. Contrast media & Molecular imaging, 10, 356-366, 2015.
- Eggen S, et al, Ultrasound-enhanced drug delivery in prostate cancer xenografts by nanoparticles stabilizing microbubbles. J Controlled Release, 187, 39-49, 2014.
- Åslund AKO, et al, Nanoparticle delivery to the brain–By focused ultrasound and self-assembled nanoparticle-stabilized microbubbles. J. Control Release, 220, 287-294, 2015.
- Sontum, PC, et al, Acoustic Cluster Therapy (ACT)–A novel concept for ultrasound mediated, targeted drug delivery. Int J Pharm, 495 1019-1027, 2015.
- Healey AJ, et al, Acoustic Cluster Therapy: In Vitro and Ex Vivo Measurement of Activated Bubble Size Distribution and Temporal Dynamics. J, Ultrasound Med Biol, 42, 1145-1166, 2016.
- van Wamel A, et al, Acoustic Cluster Therapy (ACT) – pre-clinical proof of principle for local drug delivery and enhanced uptake. J. Control Release, 224, 158-164, 2016.
- van Wamel A, et al, Acoustic Cluster Therapy (ACT) enhances the therapeutic efficacy of paclitaxel and Abraxane® for treatment of human prostate adenocarcinoma in mice. J Controlled Release, 236:15-21, 2016.
- Åslund AKO, et al, Efficient Enhancement of Blood-Brain Barrier Permeability Using Acoustic Cluster Therapy (ACT). Theranostics 7, 23-30. 2017.
