By Markus Haug, Research Scientist, Department of Clinical and Molecular Medicine.
Our immune system is powerful in fighting infection, but can also specifically kill malignant body cells such as cancer cells. When we get vaccinated, the immune system gets trained and prepared for these fights. But it is believed that vaccines might also have the potential to educate our immune system to fight cancer cells.
A major challenge is that the most effective immune cells to kill cancer cells, so-called CD8+ cytotoxic T lymphocytes (CTLs), are difficult to activate with currently available vaccination strategies. This is thought to be a major reason why we do not have more effective vaccines against cancer. In a collaboration project between the Center for Molecular Inflammation Research (CEMIR) at NTNU, Oslo University Hospital and PCI Biotech AS, we have recently published that a novel and innovative vaccination technology holds great potential to realize therapeutic vaccination against cancer.
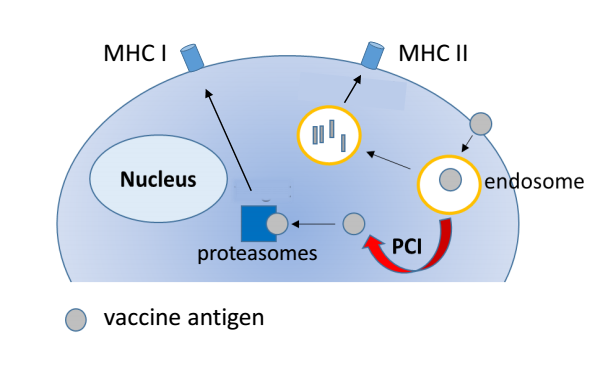
Most of today’s vaccines contain small particles with proteins from a virus or bacterium (antigens) which are recognized and taken up by immune cells (such as macrophages and dendritic cells). The vaccine particles are degraded within membrane-enclosed vesicles in the immune cells. Subsequently, small degradation fragments of the vaccine are presented on the cell surface and can be recognized by other cells of the immune system. This results for example in production of antibodies that target and inactivate bacterial or viral proteins, but does not efficiently activate CTL responses.
In our current study, we investigated the potential of a novel vaccination technology on twisting the outcome of vaccination towards increased activation of CTL responses as this is important for development of effective vaccines against cancer. “PCI vaccination” is a vaccination technology that is based on the principle of photochemical internalization (PCI), which uses a photoactive compound, a so-called photosensitizer, and light of a specific wavelength to delivers the vaccine.
In PCI vaccination, the photosensitizer compound is delivered to immune cells together with the vaccine antigen. After internalization, the photosensitizer incorporates into the membranes of the vesicles that contain the vaccine. If the immune cells are subsequently exposed to light of a specific wavelength, the photosensitizer is activated and generates a small and short-lived local damage in the membranes of the vesicles. The damage leads to rupture of the vesicles, the vaccine can escape and access the cytosolic space of the cell. This light-induced translocation of the vaccine from the vesicles to the cytosolic space of the immune cell is the key feature of PCI vaccination, since vaccines located in the cytosolic space of immune cells are very efficient in activating CTL responses.
We performed experiments with PCI vaccination technology both in a cell culture system as well as in a mouse model. We found that PCI-mediated vaccine delivery to immune cells made vaccines 30-100-fold more effective in activating CTL responses compared to vaccine-delivery without PCI technology. In addition, it was found that the PCI vaccination treatment in itself had an enhancing (“adjuvant”) effect by stimulating immune cells, probably due to the low-grade cell damage generated by the treatment. We could confirm these findings in mice by demonstrating that PCI vaccination was able to effectively induce specific CTL responses to two cancer antigens. We thus show new and compelling evidence that PCI technology may provide a feasible strategy to improve the outcome of vaccines that aim at inducing CTL responses that can fight cancer cells.
The vaccine compounds used in our study were small fragments of proteins (“peptide antigens”) derived from cancer cells, which usually are poorly immunogenic. Our findings may be of particular interest since these short peptides have attractive features for therapeutic cancer vaccination: They are generally non-toxic, cheap and easy to produce and can be tailored to the patient-specific cancer. We therefore believe that PCI-mediated vaccination may provide a promising novel approach to realize effective therapeutic vaccination against cancer by raising specific CTL responses against cancer cells found in patients. The PCI method is minimally invasive, well-tolerated and has been tested in clinical trials for other purposes. PCI Biotech currently conducts a clinical validation of the PCI vaccination technology (fimaVACC) in a Phase I (“Proof of Principle!) study in healthy volunteers.
The paper is published in the journal “Frontiers in Immunology”.