Blogger: Trude Helen Flo, co-director CEMIR
In CEMIR we will work with infection, or inflammation. Why? Because inflammation is important for health, including global health, and we believe that a better understanding of how inflammatory processes are initiated and regulated will contribute to the development of new drugs and vaccines.
Inflammation is the body’s response to danger. Cells from the immune system have receptors that recognize microbial structures, tissue damage or harmful changes in the body’s own molecules. Binding to the receptors activates immune cells and acute inflammation ensues. The cells secrete signalling molecules to recruit new immune cells and antimicrobial programmes are activated, all with the purpose of removing the intruders, repairing the damage and healing the wounds. The principles are the same, but the receptors that are activated are specific to different molecules and thus the immune response adapts to the type of attack to which the body is exposed
If the body is unable to handle or clear the insult, the inflammation can become chronic, as seen in rheumatoid arthritis, inflammatory bowel disease, some cancers and cardiovascular disease. And herein lays the key: If we know, at a molecular level, what host defense mechanisms are mobilized by immune cells in response to different attacks, we can identify new and specific therapeutic targets for a number of diseases where inflammation is central.
If we know, at a molecular level, what host defense mechanisms are mobilized by immune cells in response to different attacks, we can identify new and specific therapeutic targets for a number of diseases where inflammation is central.
What about inflammation from a global health perspective? Recent figures show that even though infectious diseases are still a major cause of death in low-income countries, populations are aging and people are dying from cardiovascular disease, lung disease, diabetes, dementia and cancer – all chronic diseases that have a significant inflammation component (Lozano et al, The Lancet 2012). Some of the most deadly infectious diseases are also chronic, such as HIV / AIDS, tuberculosis and malaria.
My research group is particularly interested in tuberculosis caused by the bacterium Mycobacterium tuberculosis. One-and-a-half million people still die from tuberculosis each year, and it is estimated that one-third of the world’s population is infected with the tubercle bacillus. Treatment is complicated and lengthy, there is an increasing incidence of resistance to the drugs we have today, and the only available vaccine, the BCG vaccine, is not effective against adult pulmonary tuberculosis. There is therefore a great need for both new vaccines and more effective drugs against tuberculosis.
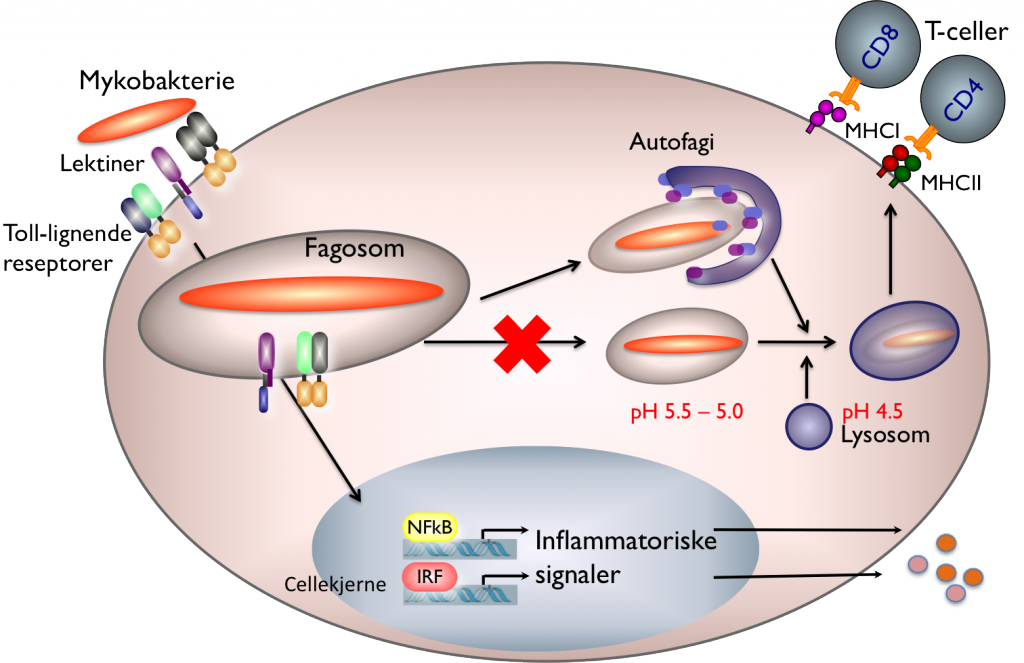
We believe we can contribute to these needs by studying the interaction between mycobacteria and the host immune system, in particular how mycobacteria are able to survive inside a host cell that is specialized in destruction of microbes, the macrophage. A number of processes are initiated in the macrophage when the mycobacteria bind to receptors on the surface (see Figure 1): lectin receptors contribute in uptake of the bacterium into the cell. This happens in a process called phagocytosis whereby the cell membrane wraps around the bacterium creating a closed vesicle, a phagosome. At the same time, the inflammatory process is activated in the macrophage via Toll-like receptors, while signalling molecules are secreted and an antibacterial programme is initiated.
But here’s what happens that makes mycobacteria special: Normally the phagosome would become gradually more acidic, the bacterium destroyed and pieces of it presented to more professional immune cells, the T cells. Pathogenic mycobacteria can prevent this process and live for a long time inside the host without being killed. This is called immune evasion, and may explain why so many people are walking around with latent tuberculosis.
Our goal is to understand the molecular mechanisms that make this possible, both in the mycobacteria and in the macrophage. If we can strengthen the macrophage or weaken the mycobacteria such that T cells are activated in a timely manner, we can hopefully provoke an immune response that effectively kills the bacteria and provides immunity. It is only through a detailed understanding of what happens between mycobacteria and the host that we can find new therapeutic targets and develop new vaccines.
Mycobacteria require iron to grow. We have shown that the body has an antibacterial protein, lipocalin 2, which starves bacteria for iron (Flo et al., Nature 2004). Unfortunately, we have also found that lipocalin 2 is not as effective as a defence against mycobacteria because the pathogen hides inside macrophages, in phagosomes, where lipocalin 2 cannot get access (Halaas et al., Journal of Infectious Diseases 2010, see Figure 2).
If we can find other ways to starve mycobacteria for iron, they will weaken enough that the macrophages will be able to take care of them. Better knowledge of mycobacterial iron metabolism may help us find a new point of attack.
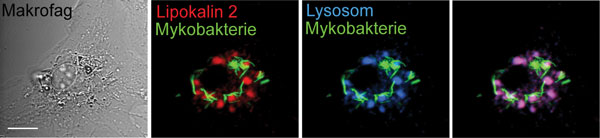
Figure 2. Mycobacteria live in the body’s macrophages and hide from defence proteins such as lipocalin 2. The pictures show a macrophage that is infected with a fluorescent mycobacteria (green) and then labelled with the defence protein lipocalin 2 (red) and lysosomes (blue). Lipocalin 2 is transported into the lysosomes where it is degraded (the overlapping red and blue becomes purple), while mycobacteria move to other parts of the macrophage, where they are neither broken down nor come into contact with lipocalin 2. This is called “immune evasion”, a survival strategy at which mycobacteria are expert. Source: Halaas et al., Journal of Infectious Diseases 2010. Photos: Øyvind Halaas
Recent research has shown that macrophages can take care of mycobacteria using an internal waste disposal system called autophagy. Autophagy is similar to phagocytosis, but occurs inside cells, when old and damaged organelles, aggregated proteins, bacteria and viruses are surrounded by an inner membrane (Figure 1). The vesicle that is formed is called an autophagosome, and the contents are destroyed by fusion with lysosomes in the same way as for normal phagocytosis.
Autophagy is regulated by inflammatory proteins and inflammation is affected by autophagy. We believe that a better understanding of these relationships can make it possible to influence these processes in a way that enhances the killing of mycobacteria, improves the activation of T-cells, and hopefully resolves the infection. Autophagy is also important in heart failure, cancer and nerve disorders: defects in autophagy can increase the aggregation of proteins, such as is seen in Alzheimer’s disease.
So is inflammation relevant to global health? Definitely. Inflammatory mechanisms are common to both infections and chronic inflammatory diseases caused by harmful changes in the body’s own molecules. But they are used differently, and at CEMIR we hope to identify the similarities and differences in the molecular mechanisms of inflammation that may eventually give us new drugs, new vaccines and new diagnostic tools.
 The official opening of the Norwegian University of Science and Technology’s four new centres of excellence (CoE) will take place on Monday 10 June. CEMIR, the Centre of Molecular Inflammation Research, is one of these new centres. CEMIR researchers will study new mechanisms that set off inflammatory responses. We hope this will provide us with information that could help in the development of new treatment methods and the diagnosis of diseases in which inflammation plays a crucial role. You can read more about CEMIR at http://www.ntnu.edu/cemir
The official opening of the Norwegian University of Science and Technology’s four new centres of excellence (CoE) will take place on Monday 10 June. CEMIR, the Centre of Molecular Inflammation Research, is one of these new centres. CEMIR researchers will study new mechanisms that set off inflammatory responses. We hope this will provide us with information that could help in the development of new treatment methods and the diagnosis of diseases in which inflammation plays a crucial role. You can read more about CEMIR at http://www.ntnu.edu/cemir
In June there will be more blogs from CEMIR researchers.