Blogger:Trude Helen Flo, co-director CEMIR
The Research Council of Norway has recently awarded grants under the funding scheme Independent Basic Research Projects – Medicine, Health Sciences and Biology (FRIMEDBIO). There is tough competition for this funding nationally, and only the best projects get through. The Faculty of Medicine, NTNU, has been awarded funding for three talented young researchers, three research projects and two post docs. You can read about all these projects on the blog over the coming weeks. Trude Helen Flo and her colleagues at CEMIR were awarded funding to their research project: New Principles of mycobacterial killing in host macrophages (MycoHosPath).
Mycobacterial infections are a global health problem. Tuberculosis (TB) is caused by a bacterium known as Mycobacterium tuberculosis (Mtb) and kills more than 1.4 million people worldwide each year. Environmental mycobacteria like Mycobacterium avium can cause disease in immunocompromised people like HIV/AIDS patients who are not on anti-retroviral treatment. Mycobacterial infections require long treatment with antibiotics and drug resistance is emerging. Thus we need new drugs and vaccines in order to reach the UN millennium goal of eradication of tuberculosis.
To discover new therapeutic targets we need to learn more about the mycobacterium and how it interacts with its human host.
To discover new therapeutic targets we need to learn more about the mycobacterium and how it interacts with its human host.
Some major scientific and technological advances during the last decade have contributed significantly to progress in the field: studying the genetic makeup of mycobacteria provides clues about how the bacteria may infect and survive in the host. On the other hand, studying genetic variations in humans also provides insights in to how humans may become susceptible to mycobacterial infections.
Major breakthroughs in how our first line of defense, the innate immune response, can discriminate between different pathogens and shape the following second line of defense, the adaptive immune response, was awarded the 2011 Nobel Prize in Medicine. The adaptive immune response is crucial for the development of immunological memory. Despite these major advances, we still lack a complete understanding of mycobacterial immunity.
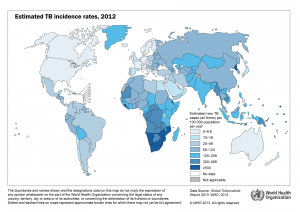
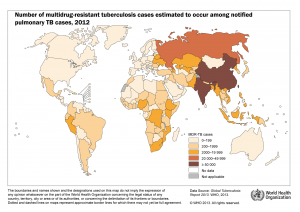
TB incidence rates and multidrug-resistant TB cases (click to enlarge image)
The primary research goal of the MycoHosPath project is to identify new principles of mycobacterial killing during acute and chronic infection. We will approach this by studying the interplay between three cellular pathways that are central for killing and intracellular survival of mycobacteria: Phagocytosis, Inflammatory signaling and Autophagy.
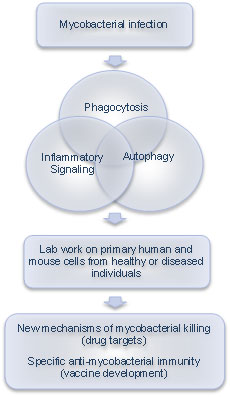 Phagocytosis is the process by which bacteria are taken up by innate immune cells like macrophages and dendritic cells. Normally this leads to destruction, but pathogenic mycobacteria have found ways to avoid it.
Phagocytosis is the process by which bacteria are taken up by innate immune cells like macrophages and dendritic cells. Normally this leads to destruction, but pathogenic mycobacteria have found ways to avoid it.
Autophagy is a similar process used by cells to detect and degrade garbage in their interior, including intracellular pathogens. Understanding how mycobacteria avoid these killing mechanisms and survive within macrophages may aid in discovery of new drug targets.
Inflammatory signaling is the macrophage response to infection. Infected macrophages produce potent molecules to alarm and recruit other immune cells to help clear the infection. Some of these molecules also help the infected cell to directly kill the invading micobes. However, since pathogenic mycobacteria can live in our body for a lifetime, this does not work perfectly. If we can improve our understanding on how a perfect immune response to mycobacteria should look like we could contribute to new vaccine strategies.
The combined processes of phagocytosis, autophagy and inflammatory signaling in host macrophages, and strategies used by the mycobacterium to manipulate them to its own advantage, will influence activation of mycobacterium-specific immune cells that we need to clear the infection and create immunity to further infection.
Our research group studies several of these aspects in the bacterium, in cells isolated from healthy and HIV-infected individuals, and in mouse model systems. As part of SFF-CEMIR we have access to national and international expertise on inflammation research and advanced imaging, state-of-the-art methodologies and new labs in Kunnskapssenteret.
We have also engaged strong national and US collaborators on autophagy and mycobacterial research who will contribute in what we hope will be a successful project. We are looking forward to realizing MycoHosPath next year.